Nyob rau hauv xyoo tas los no, kev kho mob rog rog thiab ntshav qab zib hom 2 tau dhau los ua kev hloov pauv. Ua raws li GLP-1 receptor agonists (piv txwv li, Semaglutide) thiab dual agonists (xws li, Tirzepatide),Retatrutide(LY3437943), ibtriple agonist(GLP-1, GIP, thiab glucagon receptors), tau qhia txog kev ua tau zoo uas tsis tau muaj dua. Nrog cov txiaj ntsig zoo kawg nkaus hauv kev txo qhov hnyav thiab txhim kho metabolic, nws tau suav tias yog kev kho mob zoo rau cov kab mob metabolic.
Mechanism ntawm Action
-
GLP-1 receptor activation: Txhim kho insulin secretion, suppresses qab los noj mov, qeeb plab hnyuv.
-
GIP receptor activation: Ua kom cov piam thaj txo qis ntawm GLP-1, txhim kho insulin rhiab heev.
-
Glucagon receptor activation: Txhawb kev siv hluav taws xob thiab roj metabolism.
Kev sib koom ua ke ntawm peb cov receptors tso cai rau Retatrutide kom dhau cov tshuaj uas twb muaj lawm hauv kev poob phaus thiab tswj glycemic.
Clinical Trial Data (Phase II)
Hauv ibPhase II sim nrog 338 tus neeg mob hnyav / rog, Retatrutide pom tau tias muaj txiaj ntsig zoo heev.
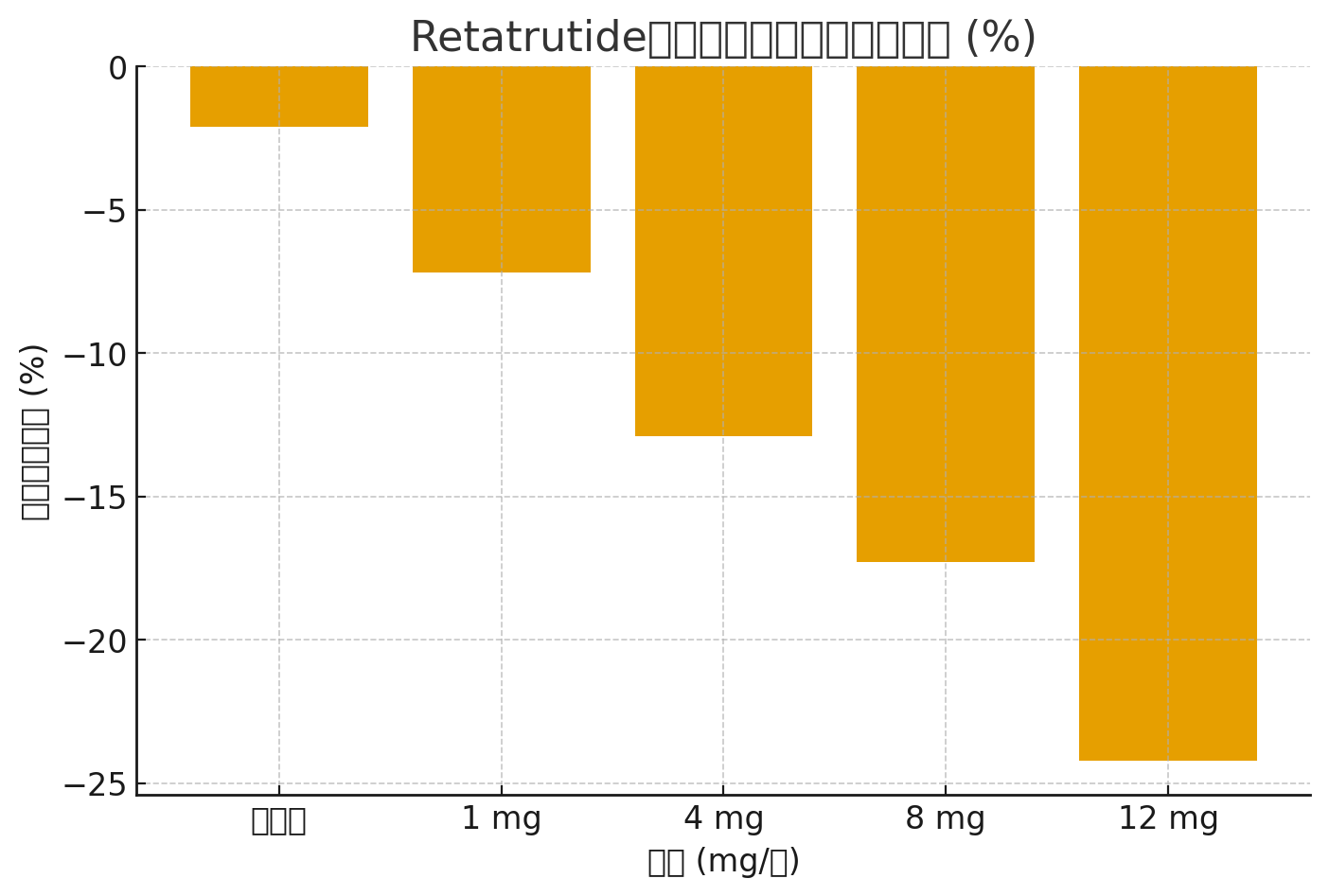
Cov lus: Kev sib piv ntawm Retatrutide vs. Placebo
| Dose (mg / lub lis piam) | Qhov hnyav txo qis (%) | Kev txo qis HbA1c (%) | Cov xwm txheej tsis zoo |
|---|---|---|---|
| 1mg ib | -7.2% | -0.9% | xeev siab, ntuav me ntsis |
| 4mg ib | -12.9% | -1.5% | xeev siab, qab los noj mov |
| 8mg ib | -17.3% | -2.0% | GI tsis xis nyob, raws plab me me |
| 12 mg ib | -24.2% | -2.2% | xeev siab, qab los noj mov, cem quav |
| Cov tshuaj placebo | -2.1% | -0.2% | Tsis muaj kev hloov pauv tseem ceeb |
Data Visualization (Qhov hnyav txo qhov sib piv)
Daim ntawv qhia bar hauv qab no qhia txog qhovQhov nruab nrab qhov hnyav txoCov koob tshuaj Retatrutide sib txawv piv nrog cov placebo:
Post lub sij hawm: Sep-16-2025